[ad_1]
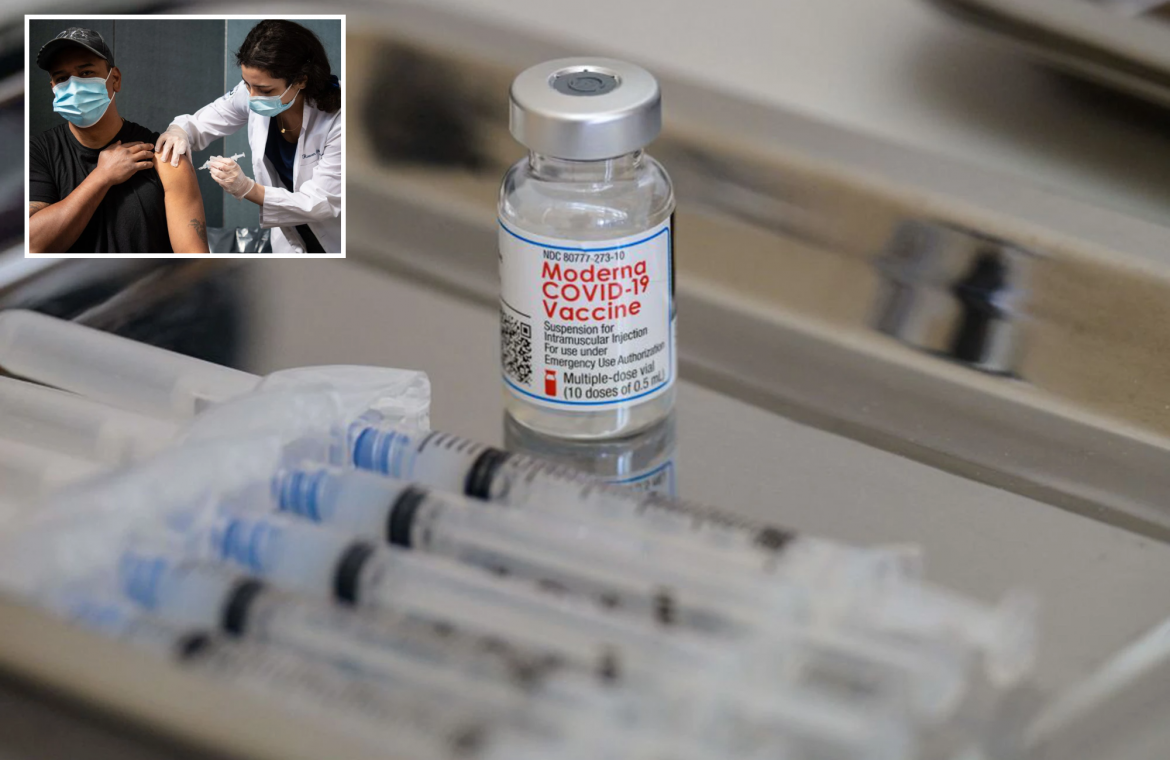
The Food and Drug Administration announced it has granted Moderna’s COVID-19 vaccine full approval for use in adults on Monday.
The mRNA-based vaccine, also known as Spikevax, has been in use in the US for over 11 months under an emergency use authorization.
“The public can be assured that Spikevax meets the FDA’s high standards for safety, effectiveness and manufacturing quality required of any vaccine approved for use in the United States,” Acting FDA Commissioner Janet Woodcock said in a statement.
The Moderna vaccine is the second COVID-19 vaccination to receive full FDA approval — the Pfizer-BioNTech vaccine was approved in August 2021.
“Our COVID-19 vaccine has been administered to hundreds of millions of people around the world, protecting people from COVID-19 infection, hospitalization and death,” Moderna CEO Stéphane Bancel said in a statement.
“We are grateful to the U.S. FDA for their thorough review of our application,” he said. “We are humbled by the role that Spikevax is playing to help end this pandemic.”
Currently, Moderna is administered only to adults in the US. Moderna said last fall the FDA had delayed deciding whether to allow the shots for 12- to 17-year-olds over concerns of a rare risk of heart inflammation affecting mostly young men and teen boys.
In its announcement Monday, the FDA said it had seen the rare risk within seven days of a second Moderna dose, specifically in men ages 18 to 24.
Moderna is the second COVID-19 vaccine to be approved for full use, the first being Pfizer.ANGELA WEISS/AFP via Getty Images
The Food and Drug Administration has approved the full use of the Moderna COVID-19 vaccine. ANGELA WEISS/AFP via Getty Images
“Available data from short-term follow-up suggest that most individuals have had resolution of symptoms,” the agency said. “However, some individuals required intensive care support.”
The FDA said Monday it was requiring Moderna to continue studying post-vaccination instances of heart inflammation.
The benefits of the Moderna vaccine outweigh the risks, the FDA said.
The town of Islip, in partnership with Good Samaritan Hospital, has launched a new vaccination site on the grounds of the Brentwood Recreation Center on July 14, 2021.Steve Pfost/Newsday RM via Getty Images
Heart inflammation is also a relatively common side effect of COVID-19 infection, according to the Centers for Disease Control and Prevention.
With Post wires
[ad_2]
Source link